A New Collaboration Finds a Match Between Liver-Derived Organoids and Automated Screening Technologies
Such work may lead to a promising leap forward for disease research and drug development
 An interesting and exciting development
at the Society of Automation and Screening (SLAS) conference this year came
from a global science and technology innovator and one of its operating
companies. Danaher Corporation announced a new collaboration with Cincinnati
Children’s Hospital Medical Center using their liver organoids and Molecular
Devices’ automated cell culture solutions to predict drug-induced liver injury
in the drug discovery process.
An interesting and exciting development
at the Society of Automation and Screening (SLAS) conference this year came
from a global science and technology innovator and one of its operating
companies. Danaher Corporation announced a new collaboration with Cincinnati
Children’s Hospital Medical Center using their liver organoids and Molecular
Devices’ automated cell culture solutions to predict drug-induced liver injury
in the drug discovery process.
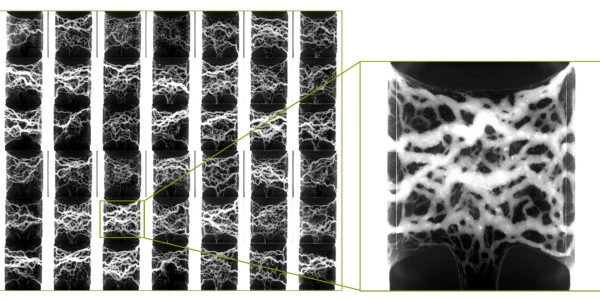
Liver organoids predict drug-induced liver injury in the preclinical phase with roughly 80 percent accuracy -- compared to the 33 to 50 percent accuracy of widely adopted in-vitro models. Predicting drug-induced liver injury with organoids could improve drug safety, accelerate the discovery of new therapies, and more quickly identify the leading cause of drug failure. The collaboration between Danaher Corporation, Molecular Devices, and the Center for Stem Cell & Organoid Medicine (CuSTOM) at Cincinnati Children’s will result in high-throughput technologies to enable scale up production of liver organoids. This includes aspirations to create a cell bank and enable better representation of human genetics and improve diversity in clinical trials.
Q&A
Molecular Devices has been a leading force in the campaign to synergize automation with organoid development and screening. We met with Shantanu Dhamija, Vice President of Strategy and Innovation, at Molecular Devices to learn more about the new collaboration and the promise these organoid screening platforms hold for future drug development and disease research.
Damon Anderson, LabX:
Molecular Devices is collaborating with the Center for Stem Cell and Organoid Medicine (CuSTOM) at Cincinnati Children’s Hospital Medical Center (CCHMC) to develop iPSC-derived liver organoids for screening applications. Can you tell us more about this initiative?
Shantanu Dhamija, Molecular Devices:
A core belief that we have for advancing organoid science is all about taking the guesswork out of generating organoids. The design of automated technologies such as the CellXpress.ai platform supports that belief. To use an analogy, you can think about this technology like an oven where you put cells in and you get organoids out. I think we are in a stage in organoid development where—extending the oven analogy—ready-to-use cake mixes are an ideal approach. To this end, we have been focused on taking it to the next level by instilling standard methods and quality control to ensure reproducibility and consistency can be achieved when scaling up for use as screening tools.
This is where the Center for Stem Cell and Organoid Medicine (CuSTOM) at Cincinnati Children’s Hospital Medicine Center comes into the mix. What makes this group unique as a partner is its position as a stem cell research institute driven by a clinical mission -- making organoids available for organoid medicine to serve pediatric patients, liver transplant patients, and others in need. In order to get to that endpoint of patient treatment, all of the issues relevant to adopting organoids in screening – scale, throughput, reproducibility, and consistency – are relevant. CuSTOM is unique in that they have innovators pioneering new methods and, once they arrive at successful protocols, they spend a fair amount of time trying to streamline and optimize reliable organoid development workflows. There is not much fanfare or academic glory in spending the efforts to make methods more efficient and scalable. Such work does, however, fall directly in line with the CCHMC mission to build clinical organoid pipelines.
We’ve been their partner for quite some time. We helped set them up with the automation they are using in their CuSTOM lab and we knew we wanted to do more with them. With the ongoing development of our own organoid technology platform, it seemed like the right thing to do to set up this collaboration to enable scalable production of liver organoids.
LabX:
As a Danaher Beacon project, the collaboration leverages automated, AI-driven, high-throughput screening solutions from Molecular Devices and iPSC-derived liver organoids from CuSTOM at CCHMC. I see there have been previous Danaher Beacon strategic partnerships, including a program on Gene Therapy Innovation with Duke University. Can you expand on the Beacon Program, how it was conceived, and what are the goals?
SD:
The Beacon projects speak to Danaher’s transformation over time. All the operating companies under the Danaher umbrella, including Molecular Devices, function independently. Danaher has seized the opportunities to leverage knowledge and skills from these internal resources to help the operating companies as a whole. They have been very successful at this and have in fact been used as a Harvard Business School case study.
As Danaher has evolved into a diversified science and diagnostics company, they have sought opportunities to invest in leading-edge technologies and science. The Beacon projects are different from typical business partnerships as they are intended to foster collaboration with academic institutions to advance research.
Another Beacon project Danaher announced recently is with the Innovative Genomics Institute at University of California Berkeley and Jennifer Doudna’s lab. With all of us in the life science industry, there is a huge component of the mission to make an impact on improving patient’s lives. That is what this Beacon is about. It is about gene therapies supporting rare disease research that will help adoption of technologies at a much larger scale in the future. Stable technologies for gene therapy development that can be applied in a much larger and more complex disease landscape.
 LabX:
LabX:
Molecular Devices recently released the new CellXpress.ai Automated Cell Culture System as an AI-driven cell culture innovation hub that automates processes, improves workflows, and makes assays more reliable and reproducible. This follows the previous release of the Organoid Development Automated Workcell that integrates ImageXpress High Content Imaging with liquid handling and microplate reader devices. Will the new liver organoids be compatible with these technologies? Will they be offered as part of the 3D Ready Organoid Expansion Service as well?
SD:
We want to meet customers where they are in their work. We envision multiple models. This includes a scenario where certain customers want the ingredients to be able to expand their own organoid cultures. We imagine there may be other customers who come to us and say, “Do you have organoids for these specific patients?” We want to be able to provide those organoids too.
LabX:
Beyond leveraging the strengths of 3D cell models and automated cell culture and screening. Can you comment on the challenges of scaling complex 3D biology?
SD:
A fair amount of work has to be done upfront in fine-tuning your methods, media, and protocols. This is a fundamental aspect of how something progresses from an innovative model into a mainstay technology. A method that has been optimized to work at a specific level on a certain patient’s cells for instance, doesn’t mean conditions won’t have to be adjusted to suit another patient. Finding the right methods and conditions that will work on a large population of patient samples is what is needed in order to address scalability issues. This is the work that CuSTOM will complete. Once finished, results must be validated to ensure cells are treated gently, not undergoing stress and spontaneously differentiating, etc. Molecular Devices will be helping by leveraging our CellXpress.ai platform and expertise.
LabX:
Where do you envision 3D Biology can take us regarding screening and drug development? What obstacles or roadblocks can potentially be solved thereby accelerating the progress towards therapeutics?
SD:
 Certain
types of liver susceptibilities, such as idiosyncratic drug-induced liver
injury, have a significant genetic component. What CuSTOM is doing is setting
up a cell bank with, for instance, patients stratified across different
ethnicities. This will serve as a basis for the screening platform with the objective
to generate organoids from low, medium, and high-risk patients and test whether
we are seeing a signal following drug treatment in vitro. Using this
approach, scientists will know going into clinical trials what they should be
looking for, how they should be setting doses, how they should be screening,
and basically how they should be mitigating risk more effectively. That is so
important here. The notion of a patient in a dish. Can you, before you go in
vivo, actually know what to expect? This is where concepts like complex
models and organoids really shine.
Certain
types of liver susceptibilities, such as idiosyncratic drug-induced liver
injury, have a significant genetic component. What CuSTOM is doing is setting
up a cell bank with, for instance, patients stratified across different
ethnicities. This will serve as a basis for the screening platform with the objective
to generate organoids from low, medium, and high-risk patients and test whether
we are seeing a signal following drug treatment in vitro. Using this
approach, scientists will know going into clinical trials what they should be
looking for, how they should be setting doses, how they should be screening,
and basically how they should be mitigating risk more effectively. That is so
important here. The notion of a patient in a dish. Can you, before you go in
vivo, actually know what to expect? This is where concepts like complex
models and organoids really shine.
Historically, things like liver toxicity had been known but not fully tracked when it comes to drug development. Liver toxicity can lead to acute liver failure, and this is irreversible damage. I think what really stood out for me back in 2020 was a clinical trial looking at two drugs that had to be stopped in Phase 2 because of fatalities. This could have been avoided had toxicity been seen at an earlier point in development. Researchers at the University of Michigan showed that if a liver model (similar to the organoid model developed at CCHMC) had been used as a screening assay, they would have seen the drug toxicity in vitro, saving significant resources and most importantly preserving patient lives.
A concept that is perhaps a bit far off is what a colleague of mine terms “Star Trek Medicine.” The idea of taking patient tissues, like tumors, making organoids with them and then testing all your therapeutics. Right now, that is a few years away. There is a whole regulatory component around that and so forth. But translational research is certainly the first step towards reaching that destination.
More information on this development:
Image credits: Molecular Devices